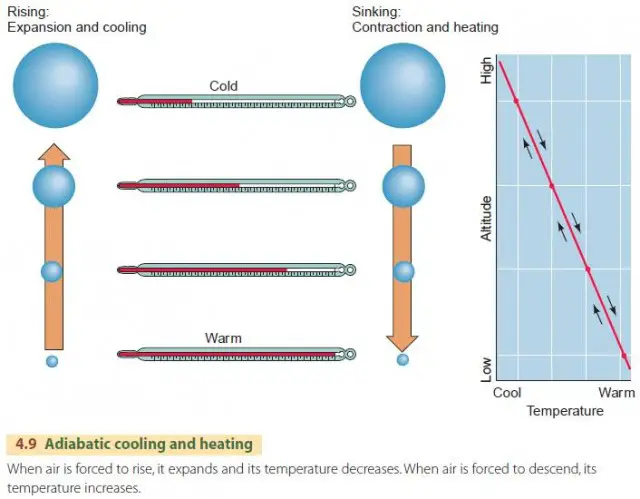
Adiabatic heating and cooling occur as air undergoes pressure changes with altitude, resulting in temperature changes. This process involves adiabatic heating from increasing pressure and adiabatic cooling from falling pressures, affecting air masses.
As air rises or sinks in the atmosphere, it expands and cools or compresses and heats up accordingly. Adiabatic heating and cooling mechanisms play a crucial role in the atmospheric processes of temperature regulation based on pressure differentials. Understanding how these processes influence the environment is essential for various fields, including climate science and meteorology.
Let’s delve deeper into the significance and applications of adiabatic heating and cooling in different natural phenomena.
Credit: www.techtarget.com
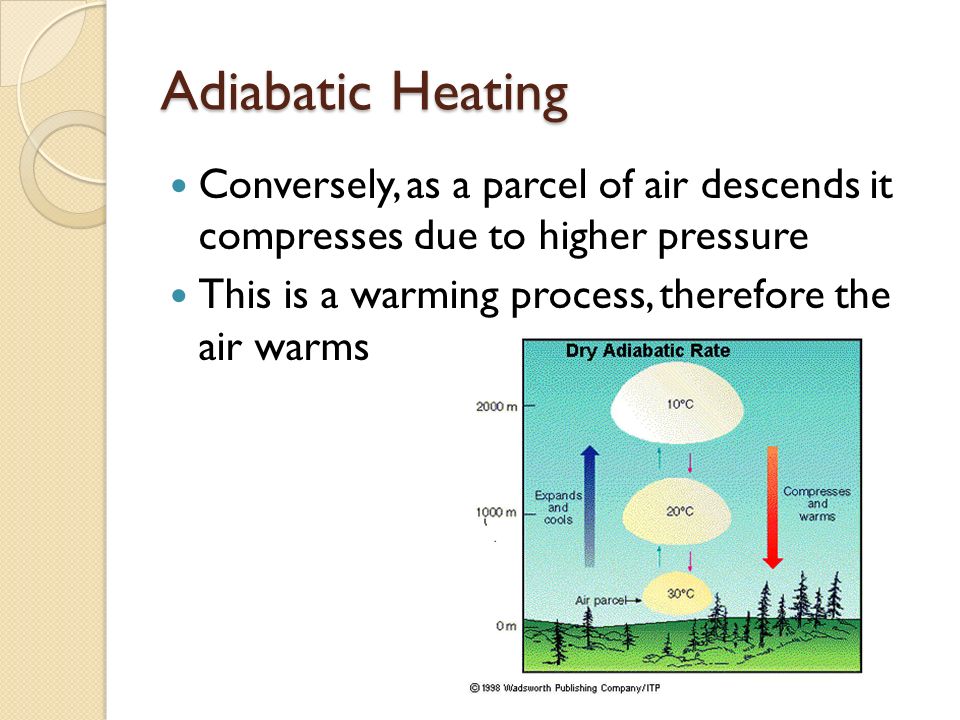
Adiabatic Heating
In the realm of thermodynamics, the concept of adiabatic heating delves into how temperature changes occur in a system without any heat exchange with the surrounding environment. The adiabatic process is crucial in understanding the behaviors of gases under pressure variations.
Causes Of Temperature Change In Adiabatic Heating
- Pressure Increase: Adiabatic heating is triggered by a rise in pressure, causing the gas to warm up as it is compressed.
- Volume Contraction: As the gas descends to lower altitudes, it experiences reduced volume leading to increased temperature.
- Rapid Compression: Processes like engine cylinder compression demonstrate adiabatic heating through swift pressure changes.
Adiabatic heating exemplifies the fascinating behavior of gases responding to alterations in pressure and volume without any external thermal influence.
Adiabatic Cooling
Understanding The Adiabatic Process
In the context of adiabatic cooling and heating, air undergoes natural temperature changes due to alterations in atmospheric pressure at various altitudes.
Real-life Examples Of Adiabatic Heat Transfer
- Compression of a gas in an engine cylinder is a rapid adiabatic process where minimal heat transfer occurs.
- The heating effect from increased pressure causes adiabatic heating as air descends towards Earth’s surface, leading to a rise in temperature.
Applications
Adiabatic Heating And Cooling In Atmospheric Currents
Adiabatic heating and cooling play a significant role in atmospheric currents. As air rises in the atmosphere, it experiences lower pressures causing it to expand and cool adiabatically. Conversely, as air descends, it encounters higher pressures, resulting in adiabatic compression and heating. This phenomenon is fundamental in the formation of clouds, winds, and weather patterns.
Adiabatic Process In Ideal Gas
The adiabatic process in ideal gas refers to the temperature changes experienced by a gas when compressed or expanded without heat exchange with the surroundings. This process obeys the first law of thermodynamics and is commonly observed in various practical applications such as diesel engines, compressors, and industrial air conditioning systems.
Credit: slideplayer.com

Credit: study.com
Frequently Asked Questions For Adiabatic Heating And Cooling
What Are Adiabatic Mechanisms Of Heating And Cooling?
Adiabatic mechanisms of heating and cooling occur when air changes altitude, resulting in changes in atmospheric pressure. Increasing pressure heats air, while decreasing pressure allows it to cool without adding or subtracting heat energy. This process is common in convective atmospheric currents.
What Is The Adiabatic Process Of Heat?
The adiabatic process of heat is when air is heated or cooled without adding or subtracting any heat energy. It occurs as air experiences changes in atmospheric pressure due to changes in altitude. Increasing pressure causes the air to heat up, while decreasing pressure allows the air to expand and cool.
This process is common in convective atmospheric currents.
Why Does Temperature Change In Adiabatic Heating?
In adiabatic heating, temperature changes due to pressure increases, causing air to compress and heat up. This occurs as air experiences atmospheric pressure changes with different altitudes. Rising pressure heats air adiabatically, while falling pressure allows air to expand and cool.
What Is An Example Of Adiabatic Heat Transfer?
An example of adiabatic heat transfer is the rapid gas compression in a cylinder of an engine, where little energy is transferred as heat to the surroundings.
How Does Adiabatic Heating And Cooling Occur In The Atmosphere?
Adiabatic heating and cooling occur in the atmosphere as air masses experience changes in atmospheric pressure associated with different altitudes. As air rises and expands, it cools adiabatically. Conversely, as air sinks and compresses, it heats adiabatically.
Conclusion
Adiabatic heating and cooling play a vital role in environmental processes and industrial applications. Understanding the principles of adiabatic mechanisms is crucial in managing temperature changes in various systems. As we continue to explore these phenomena, the potential for innovative solutions in heating and cooling technologies becomes increasingly apparent.